Magnetic materials:In this post, we will discuss the type of magnetic materials namely diamagnetic, paramagnetic, ferromagnetic, antiferromagnetic, and ferrimagnetic materials.
Download & Install EEE Made Easy App
Paramagnetic, Ferromagnetic, Antiferromagnetic, & Ferrimagnetic Materials
Magnetic Basics:
The response of a material, when subjected to an external magnetic field, is the root of magnetism.
Method of Magnetization can be read HERE
The spinning electrons in the material behave like tiny magnets.
These tiny magnets are aligned in the direction of the applied magnetic field and thereby the material is magnetized.
Join EEE Made Easy Whatsapp Channel
Types of Magnetic Materials
The orbital and spin motion of of electrons and interaction between these electrons is the origin of magnetism.
Different types of magnetic materials are due to differences in their response to external magnetic fields.
Some materials are much more magnetic than others. why?
The reason is in some materials there is a strong interaction between the atomic magnets, whereas in other materials there is no interaction between the atomic magnets.
Depending upon the magnetic behavior of materials, they can be classified into the following five major groups:
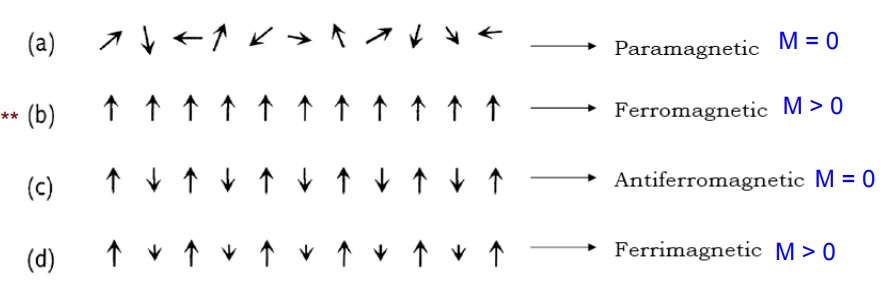
1.DIAMAGNETIC SUBSTANCES
2.PARAMAGNETIC SUBSTANCES
3.FERRO MAGNETIC SUBSTANCES
4.FERRI MAGNETIC SUBSTANCES
5.ANTI FERRO MAGNETIC SUBSTANCES
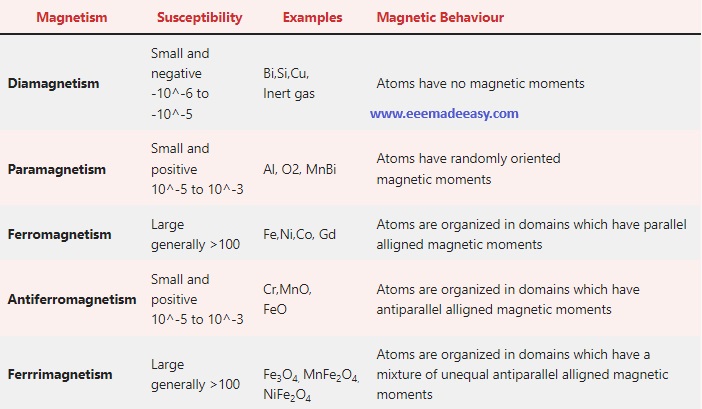
The comparison table is given below. Then you can read about each one in detail.
difference between dia para ferro antiferro and ferri magnetism
| Magnetism | Susceptibility | Examples | Magnetic Behaviour |
|---|---|---|---|
| Diamagnetism | Small and negative -10^-6 to -10^-5 | Bi,Si,Cu, Inert gas | Atoms have no magnetic moments |
| Paramagnetism | Small and positive 10^-5 to 10^-3 | Al, O2, MnBi | Atoms have randomly oriented magnetic moments |
| Ferromagnetism | Large generally >100 | Fe,Ni,Co, Gd | Atoms are organized in domains which have parallel alligned magnetic moments |
| Antiferromagnetism | Small and positive 10^-5 to 10^-3 | Cr,MnO, FeO | Atoms are organized in domains which have antiparallel alligned magnetic moments |
| Ferrrimagnetism | Large generally >100 | Fe3O4, MnFe2O4, NiFe2O4 | Atoms are organized in domains which have a mixture of unequal antiparallel alligned magnetic moments |
Diamagnetism
Diamagnetism is a weak magnetism and is the fundamental property of all matter.
Diamagnetism is mainly due to the non-cooperative behavior of the orbital electrons under the application of an external magnetic field.
In diamagnetic substances, all the atoms have paired electrons and there are no unpaired electrons in the shells.
Thus the net magnetic moment of the atom of a diamagnetic substance is zero.
However, when an external magnetic field is applied on these substances these materials are magnetized opposite to the field direction.
Thus they have negative magnetization.
That means for diamagnetic substances the susceptibility is negative.
plot M vs. H,
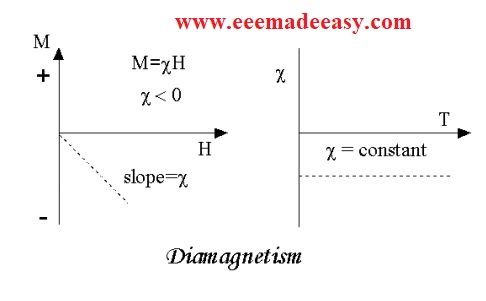
From the above plot it can be understood that the magnetization is zero when the applied is zero.
The other characteristic behavior of diamagnetic substances is that their susceptibility is independent of temperature.
Download & Install EEE Made Easy App
Paramagnetism
In the Paramagnetic materials, the atoms or ions have unpaired electrons in partially filled orbitals.
That means each atom in a paramagnetic substance has a small net magnetic moment.
But, there is no interaction between these atomic magnets.
In the presence of an external magnetic field there will be a partial alignment of these atomic magnetic moments in the direction of applied magnetic field resulting in a net positive magnetization and positive susceptibility.
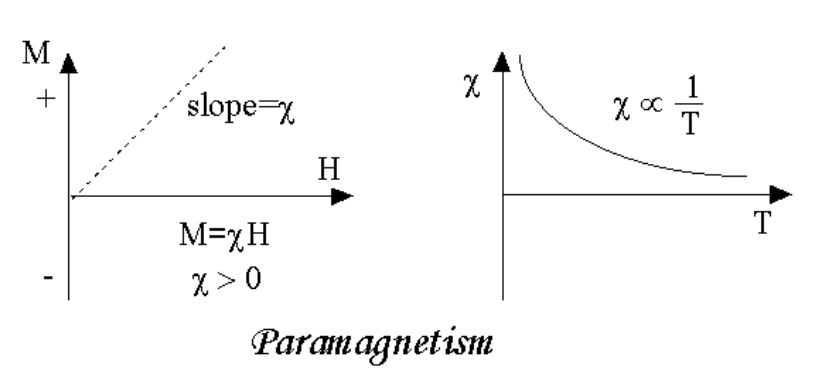
When the applied field is zero, the magnetization also becomes zero.
If the temperature of the paramagnetic substance increases, then the alignment of the atomic magnets will be disturbed.
That means the magnetic susceptibility depends on temperature.
curies law.
The magnetic susceptibility of is inversely proportional to the absolute temperature. This law is called curies law.
When a moderate magnetic field is applied on a paramagnetic substance, at room temperature, then the susceptibility is inversely proportional to the absolute temperature.
But, still it is greater than the susceptibility of a diamagnetic substance.
If the temperature of a paramagnetic substance is brought to a low temperature e(<<100K) or the magnetic applied on the subastance is very high, then the susceptibility of the paramagnetic substance does not depend on the applied magnetic field.
In such situation, the susceptibility of the paramagnetic substance depends on the total iron content in the substance.
At room temperature, most of the minerals containing iron are paramagnetic.
Superparamagnetism
Superparamagnetism is an interesting phenomenon that comes into play when ferromagnetic or ferromagnetic particles become very small.
At particle sizes of about 10 nanometers, these materials begin to exhibit paramagnetic behavior, even when they are below their curie temperature.
This is because ,below curie temperature, the thermal agitations are not strong enough .The interaction forces between the individual atoms dominate the thermal agitations.
But, the thermal agitations succeed in changing the direction of magnetization of the entire particle. As a result , the directions of magnetic moments of the particles in the crystal are arranged randomly.
Thus the net magnetic moment is zero,
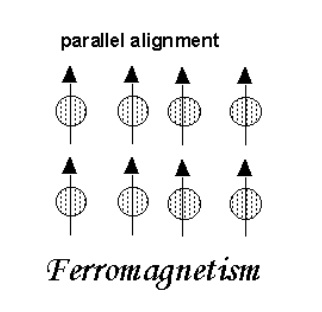
Ferromagnetism
When we think of magnetic materials , the most common materials that come into our mind are iron, nickel,and magnetite.
These are generally called ferromagnetic substances. In these substances, there exists a strong interaction between the atomic magnets.
These interaction forces are exchange type of forces.
The interaction force between the atoms is due to exchange of electrons.
The exchange type of forces are very large and are of magnitude 1000 Tesla.
This strength is almost 10^8 times the strength of the earth’s magnetic field.
Atomic magnets are aligned parallel to each other under the influence of these exchange forces.
A spinning electron behaves as a tiny magnet.
In ferro magnetic materials, the spins of the two neighboring electrons are oriented such that a strong interaction develops between the atoms containing these electrons.
This is a quantum mechanical effect.
That’s the reason why these atomic magnets are aligned parallel to each other , even in the absence of external ,magnetic field.
The peculiar characteristics of ferromagnetic materials are
a)Spontaneous magnetization
b)Crirical temperature(also called curie temperature)
Spontaneous Magnetization
The net magnetization existing in a uniformly magnetized microscpic volume under the absence of external magnetic field.
The magnitude of spontaneous magnetization depends on the spin magnetic moments of spinning electrons.
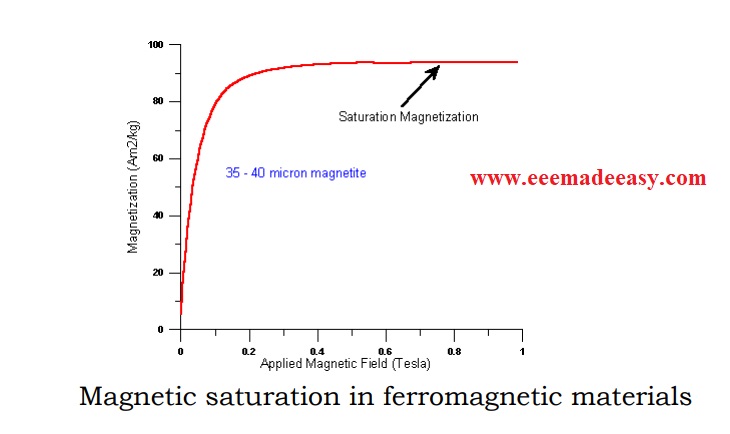
The measurable property corresponding to sponatneous magnetization is saturation magnetization.
The saturation magnetization is the maximum, induced magnetic moment that can be induced by a magnetic field (H sat); beyond this field there will be no further increase in the magnetization.
The difference between spontaneous magnetization and the saturation magnetization has to do something with magnetic domains.
Saturation magnetization is an intrinsic property, that is independent of particle size but is dependent on temperature.
Paramagnetic susceptibility is slightly greater than 1 and is positive but, ferromagnetic susceptibility is high and positive.
When compared with paramagnetic materials, the magnetization in ferromagnetic materials is going to be saturated in moderate magnetic fields ,at high (room-temperature) temperatures:
Curie temperature
In ferromagnetic substances, at a particular temperature called curie temperature, thermal agitations overcome the electronic exchange interaction energy between the atomic magnets.
This produces a randomizing effect.
Above curie temperature ,the ferromagnet is disordered.
But below curie temperature, it is ordered.
The saturation magnetization becomes zero at the Curie temperature. Fig above shows a graph between the magnetization and applied magnetic field.
The curie temperature is an intrinsic and characteristic property of the given substance.
Hysteresis
In addition to the Curie temperature, saturation magnetization, ferromagnets can retain certain amount of magnetism, even after the removal of the applied field.
This behavior is called hysteresis and graph between the variation of magnetization with appled magnetic field is called a hysteresis curve.
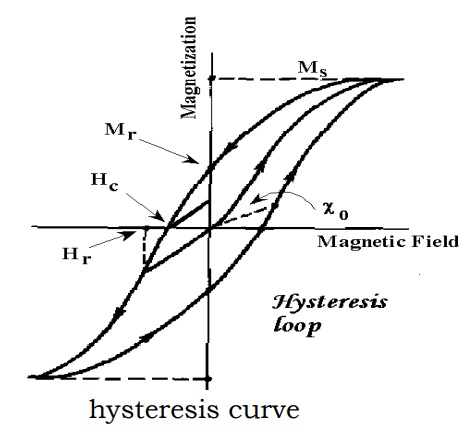
The amount of magnetism remaining in the ferromagnetic sample, when the applied field is zero, is called retentivity.
The reverse magnetic field that should be applied on the ferromagnetic sample to reduce the saturation magnetization to zero is called coercivity.
Hysteresis parameters like retentivity and coercivity etc, are not purely intrinsic properties.
They depend on the grain size, domain state, stress, temperature.
That means these hysteresis parameters are useful in magnetic grain sizing of natural samples.
Ferrimagnetism
In some ionic compounds (certain oxides of Fe) a complex form of magnetic ordering is observed due to crystal structure of those oxides.
One such type of magnetic ordering is called ferrimagnetism.
A simple orientation of magnetic spins in a ferrimagnetic oxide is shown in Fig.
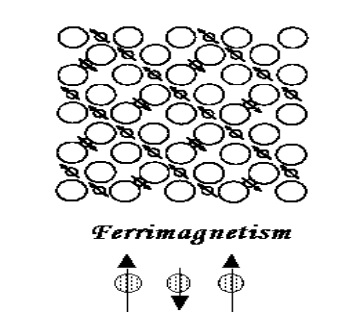
The magnetic structure in a ferrimagnetic oxide consists of two magnetic sub lattices separated by oxygen.
The exchange interaction between the two sub lattices are communicated by oxygen anions and these interactions are called super exchange interactions.
As a result of these super exchange interactions the spins of the sub lattices are aligned antiparallel.
But, magnetic moments of sub lattices are not equal.
Therefore there will be net magnetic moment.
Ferrimagnetic substance also exhibits spontaneous magnetization, Curie temperature, Hysteresis and Retentivity
Antiferromagnetism
If the magnetic moments of A and B sub lattices shown in figure below are equal in magnitude and opposite in direction, the net magnetic moment is zero.
This type of magnetic alignment is called antiferromagnetism.
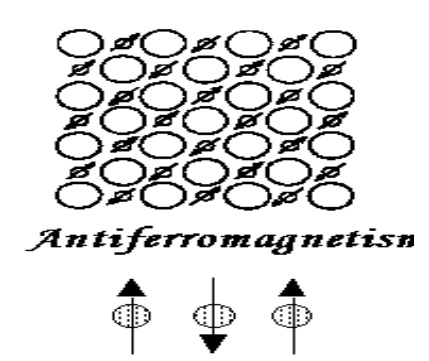
The main reason for antiferromagnetism is the behavior of susceptibility above certain critical temperature, called the Néel temperature ,denoted by (TN).
The susceptibility of paramagnetic substances obeys the Curie-Weiss law but with a negative intercept indicating the presence of negative exchange interactions.
Conclusion dia para ferro magnetic materials
The main difference between dia para ferro antiferro and ferri magnetic materials are based on the magnetic moments. Diamagnetic materials atoms have no magnetic moments .Paramagnetic materials atoms have randomly oriented magnetic moments. Ferromagnetic materials atoms are organized in domains which have parallel alligned magnetic moments .Antiferromagnetic materials atoms are organized in domains which have antiparallel alligned magnetic moments. Ferrrimagnetic materials atoms are organized in domains which have a mixture of unequal antiparallel alligned magnetic moments.
Methods of Magnetisation
Magnetism-Methods of Magnetization
Magnetism
Hope this post helped you. I hope you will share this to your friends also. Sharing is caring 🙂
Also check the blog for more notes EEE Notebook
More on Magnetic field
- 3 Effects of Electric Current|Magnetic Effect, Heating Effect & Chemical Effects
- Magnetic field MCQ|Magnetism MCQ Questions & Answers
- Magnetic data storage|Magnetic Bubble Memory, Magnetic Tapes, Magnetic Disks
- Magnetic materials: Dia, Para,Ferro,Ferri and Antiferro magnetic materials
- Magnetism-Methods of Magnetization
- What is magnetic field and its significance?|Magnetic Field
- Environment MCQ for RRB JE CBT 2|Objective Questions Environment for Competitive Exams

- RRB JE CBT 2 Computer Awareness Book Arihant|Objective Computer Awareness Book 2025

- RRB JE CBT 2 Exam Date 2025 Postponed|RRB JE CBT 2 Exam Date

- [PDF]RRB JE Result 03/2024 Cut off, Selected no of candidates for all regions

- [PDF]Final Answer Key Junior Instructor Mechanic Agricultural Machinery|643/2023 Solved Question paper





1 thought on “Magnetic materials: Dia, Para,Ferro,Ferri and Antiferro magnetic materials”