SI Units
SI – International System: The International System of Units, or SI,is a decimal and metric system of units established in 1960 and periodically updated since then.
The SI was established and is maintained by the General Conference on Weights and Measures (CGPM).
SI Units Classification
SI, the international system of units is divided into three classes :
1. Base units
2. Derived units
3. Supplementary units
Download & Install EEE Made Easy App
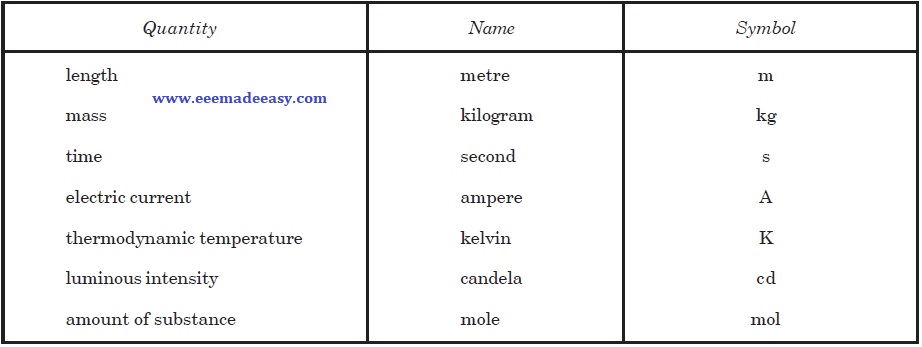
Base Units
Derived Units
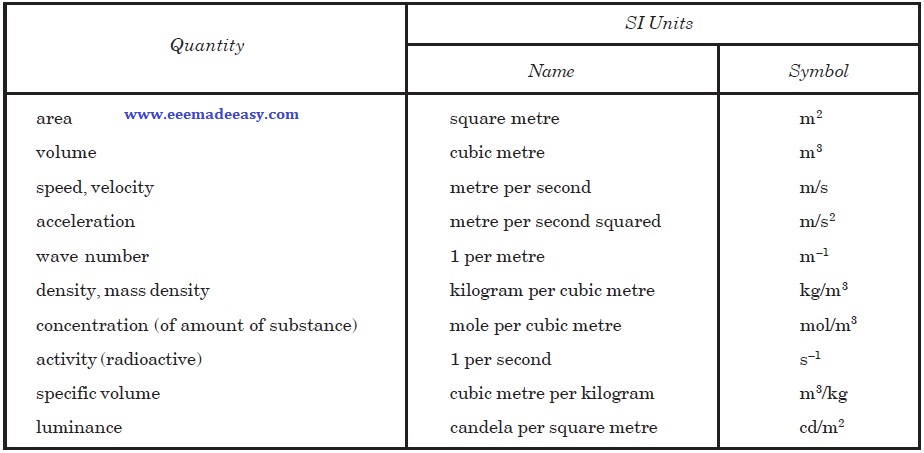
The second class of SI units contains derived units, i.e., units that can be formed by combining base units according to the algebraic relations linking the corresponding quantities.
Several of these algebraic expressions in terms of base units can be replaced by special names and symbols can themselves be used to form other derived units.
Derived units may, therefore, be classified under three headings. Some of them are given in Tables
Examples of SI Derived Units Expressed in terms of Base Units
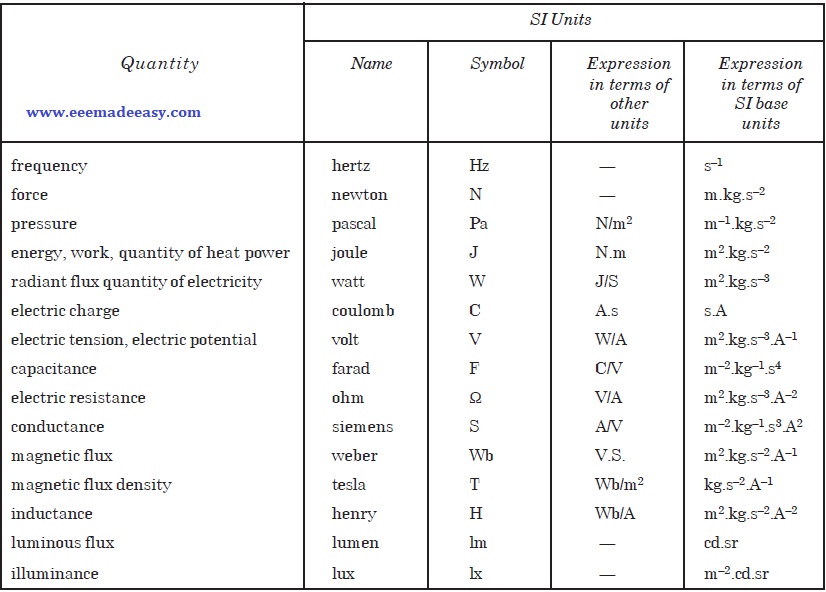
SI Derived Units with Special Names
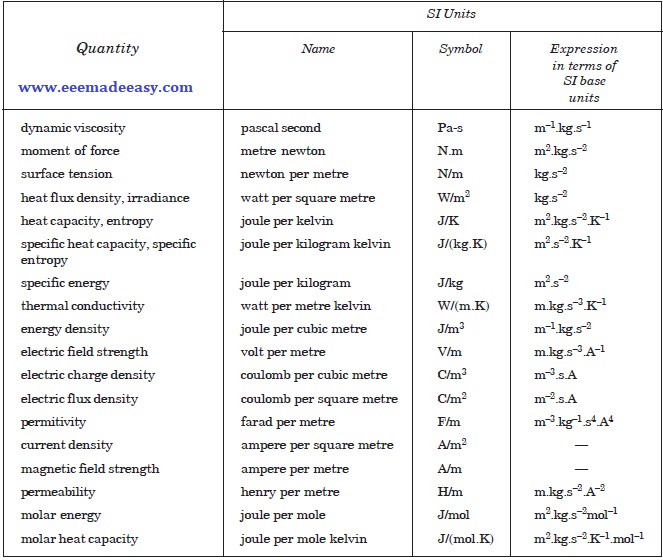
Examples of SI Derived Units Expressed by means of Special Names
The SI units assigned to third class called “Supplementary units” may be regarded either as base units or as derived units.
SI Supplementary Units

Examples of SI Derived Units Formed by Using Supplementary Units
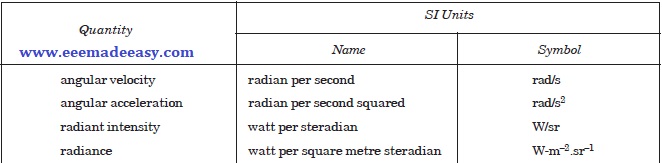
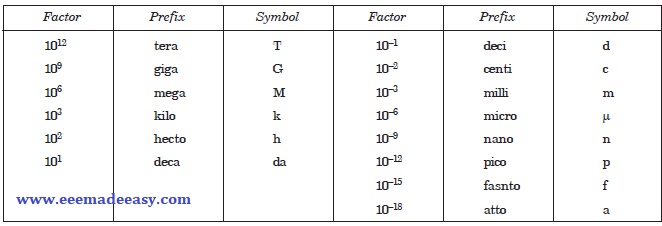
SI Prefixes
Definitions of SI Units
The SI seven base units and two supplementary units are defined below :
(i) Metre. The metre is the length equal to 1,650, 76373 wavelengths in vacuum of the radiation corresponding to the transition between the levels 2p10 and 5d5 of the krypton- 86 atom.
(ii) Kilogram. One kilogram is equal to the mass of the international prototype of the kilogram.
(iii) Second. The second is the duration of 9,192,631,770 periods of the radiation corresponding to the transition between the two hyperfine levels of the ground state of the cesium-133 atom.
(iv) Ampere. One ampere is that constant current which, if maintained in two straight parallel conductors of infinite length, of negligible circular cross-section and placed one metre apart in vacuum, would produce between these conductors a force equal to 2 × 10–7 newton per metre of length.
(v) Kelvin. The kelvin is the fraction 1/273.16 of the thermodynamic temperature of the triple point of water.
(vi) Candela. The candela is the luminous intensity, in the perpendicular direction, of a surface of a 1/600,000 square metre of a black body at a temperature of freezing platinum under a pressure of 101, 325 newton per square metre.
(vii) Mole. The mole is the amount of substance of a system that contains as many elementary entities as there are atoms in 0.012 kg, carbon 12. When the mole is used, the elementary entities must be specified and may be atoms, molecules, ions, electrons, other particles or specified groups of such particles.
(viii) Radian. The radian is the plane angle between two radii of a circle that cut off on the circle an arc equal in length to the radius.
(ix) Steradian. The steradian is the solid angle which having its vertex in the centre of a sphere, cuts off an area of the surface of the sphere equal to that of a square with sides of length equal to the radius of the sphere.
Some other Definitions of SI Units
Newton
The newton (N) is a derived unit of force and is defined as the unit of force which when acting on a mass of 1 kilogram gives it an acceleration of one metre per second.
Since acceleration due to gravity equals 9.81 m/s2, one kilogram force equals 9.81 newtons.
Joule
The joule (J) is a derived unit of energy, work or quantity of heat and is defined as the work done when a force of one newton acts so as to cause a displacement of one metre.
Energy is defined as the capacity to do work.
Electron Volt (eV)
A unit of energy in nuclear physics is the electron volt (eV) which is defined as the energy gained by an electron in rising through a potential difference of one volt.
1 eV = 1.6021 × 10–19 J.
Watt. The watt (W) is a unit of power (i.e., rate of doing work)
Power in watts = work (or energy) in joules time in seconds
Thus 1 watt equals 1 Joule/sec.
1 kilo watt-hour (kWh) = 1000 watt-hours = 36000000 joules.
Coulomb.
The coulomb (C) is the derived unit of charge.
It is defined as the quantity of electricity passing a given point in a circuit when a current of 1 A is maintained for 1 second.
Q = l.t
where Q = charge in coulombs,
l = current in amperes, and
t = time in seconds.
l coulomb represents 6.24 × 1018 electrons.
Ohm
The ohm (Ω) is the unit of electric resistance and is defined as the resistance in which a constant current of 1 A generates heat at the rate of 1 watt.
Siemen
The siemen is a unit of electric conductance (i.e., reciprocal of resistance).
If a circuit has a resistance of 5 ohms, its conductance is 0.2 siemens. A more commonly used name for siemen is mho ().
Volt
The volt is a unit of potential difference and electromotive force. It is defined as the difference of potential across a resistance of 1 ohm carrying a current of 1 ampere.
Hertz
The hertz (Hz) is a unit of frequency. 1 Hz = 1 cycle per second.
Horse-power
It is a practical unit of mechanical output. BHP (British horse power or brake horse power) equals 746 watts. The metric horse power equals 735.5 watts.
To avoid confusion between BHP and metric horse power, the mechanical output of machines in SI units, is expressed in watts or kilowatts.
Features of SI units
The salient features of SI units are as follows :
1. It is a coherent system of units, i.e., product or quotient of any two base quantities results in a unit resultant quantity.
For example, unit length divided by unit time gives unit velocity.
2. It is a rationalised system of units, applicable to both, magnetism and electricity.
3. It is a non-gravitational system of units. It clearly distinguishes between the units of mass and weight (force) which are kilogram and newton respectively.
4. All the units of the system can be derived from the base and supplementary units.
5. The decimal relationship between units of same quantity makes possible to express any small or large quantity as a power of 10.
6. For any quantity there is one and only one SI unit. For example, joule is the unit of energy of all forms such as mechanical, heat, chemical, electrical and nuclear.
However, kWh will also continue to be used as unit of electrical energy.
Advantages of SI Units
1. Units for many different quantities are related through a series of simple and basic relationship.
2. Being an absolute system, it avoids the use of factor ‘g’ i.e., acceleration due to gravity in several expressions in physics and engineering which had been a nuisance in all numericals in physics and engineering.
3. Being a rationalised system, it ensures all the advantages of rationalised MKSA system in the fields of electricity, magnetism, electrical engineering and electronics.
4. Joule is the only sole unit of energy of all forms and watt is the sole unit of power hence a lot of labour is saved in calculations.
5. It is a coherent system of units and involves only decimal co-efficients. Hence it is very convenient and quick system for calculations.
6. In electricity, all the practical units like volt, ohm, ampere, henry, farad, coulomb, joule and watt accepted in industry and laboratories all over the world for well over a century have become absolute in their own right in the SI system, without the need for any more practical units.
Disadvantages of SI Units
1. The non-SI time units ‘minute’ and ‘hour’ will still continue to be used until the clocks
and watches are all changed to kilo seconds and mega seconds etc.
2. The base unit kilogram (kg) includes a prefix, which creates an ambiguity in the use of multipliers with gram.
3. SI units for energy, power and pressure (i.e., joule, watt and pascal) are too small to be expressed in science and technology, and, therefore, in such cases the use of larger units, such as MJ, kW, kPa, will have to be made.
4. There are difficulties with regard to developing new SI units for apparent and reactive energy while joule is the accepted unit for active energy in SI systems.
Conversion factors of SI Units
1. Force :
1 newton = kg-m/sec2 = 0.012 kgf
1 kgf = 9.81 N
2. Pressure :
1 bar = 750.06 mm Hg = 0.9869 atm = 105 N/m2
= 103 kg/m-sec2
1 N/m2 = 1 pascal = 10–5 bar = 10–2 kg/m-sec2
1 atm = 760 mm Hg = 1.03 kgf/cm2 = 1.01325 bar
= 1.01325 × 105 N/m2
3. Work, Energy or Heat :
1 joule = 1 newton metre = 1 watt-sec
= 2.7778 × 10–7 kW-h = 0.239 cal
= 0.239 × 10–3 kcal
1 cal = 4.184 joule = 1.1622 × 10–6 kWh
1 kcal = 4.184 × 103 joule = 427 kgf m
= 1.1622 × 10–3 kWh
1 kWh = 8.6042 × 105 cal = 860 kcal
= 3.6 × 106 joule
4. Power :
1 watt = 1 joule/sec = 0.860 kcal/h
1 h.p. = 75 m kgf/sec = 0.1757 kcal/sec = 735.3 watt
1 kW = 1000 watts = 860 kcal/h
5. Specific heat :
1 kcal/kg-°K = 0.4184 joules/kg-K
6. Thermal conductivity :
1 watt/m-K = 0.8598 kcal/h-m-°C
1 kcal/h-m-°C = 1.163 watt/m-K = 1.163 joules/s-m-K.
7. Heat transfer co-efficient :
1 watt/m2-K = 0.8598 kcal/m2-h-°C
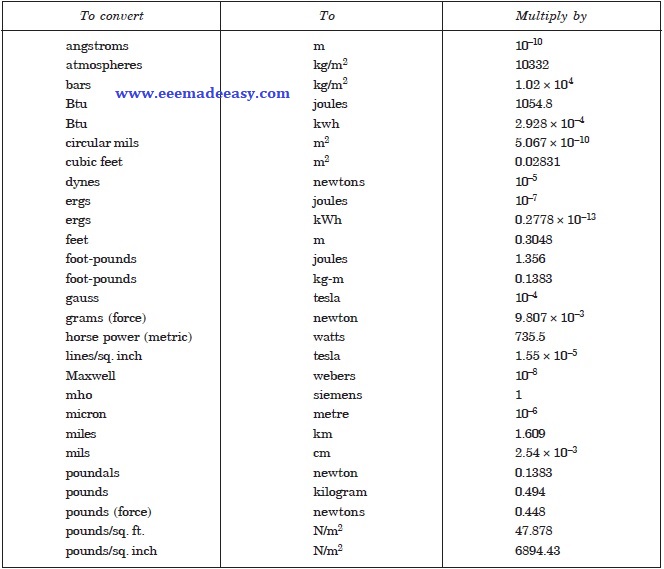
The following conversion factors may be used to convert the quantities in non-SI units into SI units.
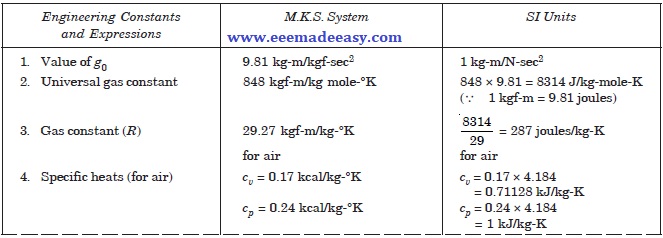
Important Engineering Constants And Expressions

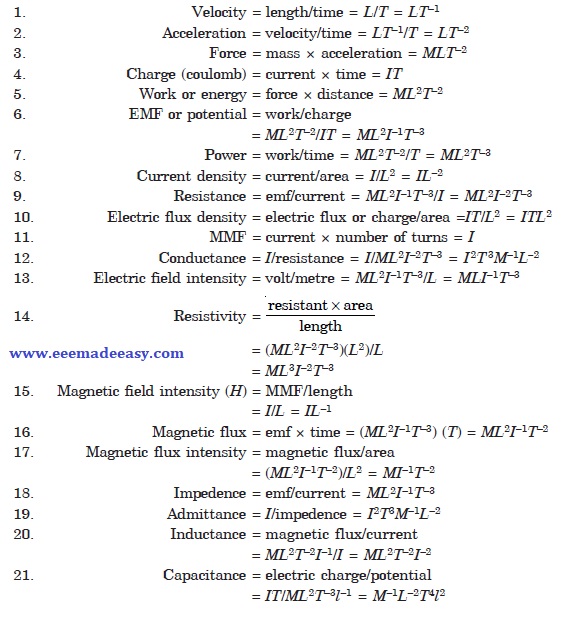
Dimensions of quantities
Different units can be represented dimensionally in terms of units of length L, mass M, time T and current
l. The dimentions can be derived as under
Download & Install EEE Made Easy App
Join EEE Made Easy Telegram channel
- Environment MCQ for RRB JE CBT 2|Objective Questions Environment for Competitive Exams
- RRB JE CBT 2 Computer Awareness Book Arihant|Objective Computer Awareness Book 2025
- RRB JE CBT 2 Exam Date 2025 Postponed|RRB JE CBT 2 Exam Date
- [PDF]RRB JE Result 03/2024 Cut off, Selected no of candidates for all regions
- [PDF]Final Answer Key Junior Instructor Mechanic Agricultural Machinery|643/2023 Solved Question paper



